(UTV | COLOMBO) – India’s Supreme Court has sharply criticised the federal government over its coronavirus vaccination programme.
The judges asked the government to explain why it was mandatory to register on an app for getting a jab.
The court said this would hamper vaccinations across rural India where internet access is difficult.
The judges also questioned whether federal policy was making individual states compete against each other for vaccines.
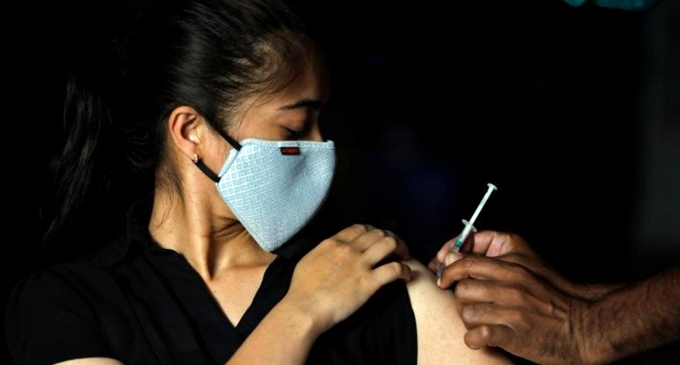
India has administered more than 220 million doses since it began vaccinating in mid-January, but so far only 3% of the population has been fully vaccinated.
Prime Minister Narendra Modi’s government has opened up vaccinations for some 960 million eligible Indians without having anything close to the required supply.
The shortage came amid a deadly second Covid wave and warnings of an impending third wave. The wave has since slowed – the number of daily infections has fallen from the peak of over 400,000 last month to 132,788 cases that were recorded in the past 24 hours.
The Supreme Court said that asking people between 18-44 years to pay for their jabs was “arbitrary and irrational”.
The court asked the government to review its vaccination policy and “place on record a roadmap of projected availability of vaccines till 31 December” – the date by which the government is promising to vaccinate the entire adult population.
India says it aims to ramp up vaccine production and has pledged to produce at least two billion doses between August and December.
There are currently two locally-made vaccines for the coronavirus: Covishield and Covaxin.
The Serum Institute of India (SII) makes Covishield (under licence from AstraZeneca), whilst the second largest producer, Bharat Biotech, makes the locally-developed Covaxin.
The Sputnik V vaccine, which was approved for use in April, is also now available, with three million doses supplied by Russia.
Of the eight vaccines currently under production in India, only three have been approved for use – another two are in the early stages of clinical trials and a further three are in late-stage trials.
Public health experts say poor planning, piecemeal procuring and unregulated pricing by the government has turned India’s vaccine drive into a deeply unfair competition for state governments.